About N-Nitrosodimethylamine (NDMA) | Ias Banenge
Context:
The Gujarat High Court recently issued a notice to the Drug Controller General of India (DCGI), in a public interest litigation (PIL) seeking immediate removal of drugs and medicines containing “cancerous impurities” such as NDMA from the Indian market
Relevance:
Facts for prelims
READ MORE – What is Saurashtra Tamil Sangamam? Ias Banenge
Dimensions of the Article:
- About N-nitrosodimethylamine (NDMA)
- About Drug Controller General of India
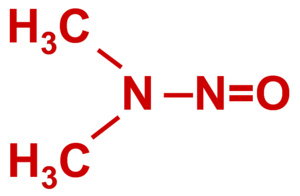
About N-nitrosodimethylamine (NDMA)
- NDMA is a volatile organic chemical that occurs widely in the environment due to its formation from common precursors.
- It is classified as a probable human carcinogen based on animal studies.
- NDMA is a yellow, odourless liquid chemical once used to make rocket fuel.
- It is a byproduct of several manufacturing processes and water chlorination.
- NDMA breaks down when exposed to sunlight.
- Exposure to NDMA can occur through food and water, as chemicals found in them can turn into NDMA in the stomach.
- Foods such as cured meats, beer, fish, cheese, and vegetables may contain NDMA.
- Some people may have had exposure to NDMA through the use of contaminated medications.
About Drug Controller General of India
The position of DCGI was established in 1962 under the Drugs and Cosmetics Act, 1940, which governs the manufacture, distribution, and sale of drugs and cosmetics in India.
Responsibilities: The DCGI is responsible for the following tasks:
- Granting licenses to manufacture drugs and cosmetics
- Regulating the import and export of drugs and cosmetics
- Monitoring and enforcing compliance with drug safety and efficacy standards
- Approving clinical trials of drugs and vaccines
- Issuing guidelines and regulations related to drugs and cosmetics
- Coordinating with other government agencies on matters related to drug regulation
Structure:
- The DCGI is part of the Central Drugs Standard Control Organization (CDSCO), which is under the purview of the Ministry of Health and Family Welfare.
- The CDSCO consists of several departments, including the Drug Control Department, the Medical Device Department, and the Biological Division, all of which work together to regulate drugs and medical devices in India.
-Source: Indian Express




Pingback: An Upswing in Rural Entrepreneurship- Ias Banenge - Ias Banenge